Medical Information
Making use of state-of-the-art technology to ensure patient safety in Europe, CIS & the US.
Helping to guarantee patient safety. Twenty-four hours a day.
A trusted, highly engaged MI partner for Pharma, Biotech, and Medical Device companies, delivering excellence and ensuring compliance across Europe, CIS, and the US throughout the product lifecycle. We offer a spectrum of Medical Information (MI) Services delivered on a 24/7 basis by our bilingual native-speaking professionals. Biomapas’ experienced Medical Information experts employ state-of-the-art technologies to help you produce reliable information efficiently and help you guarantee patient safety.
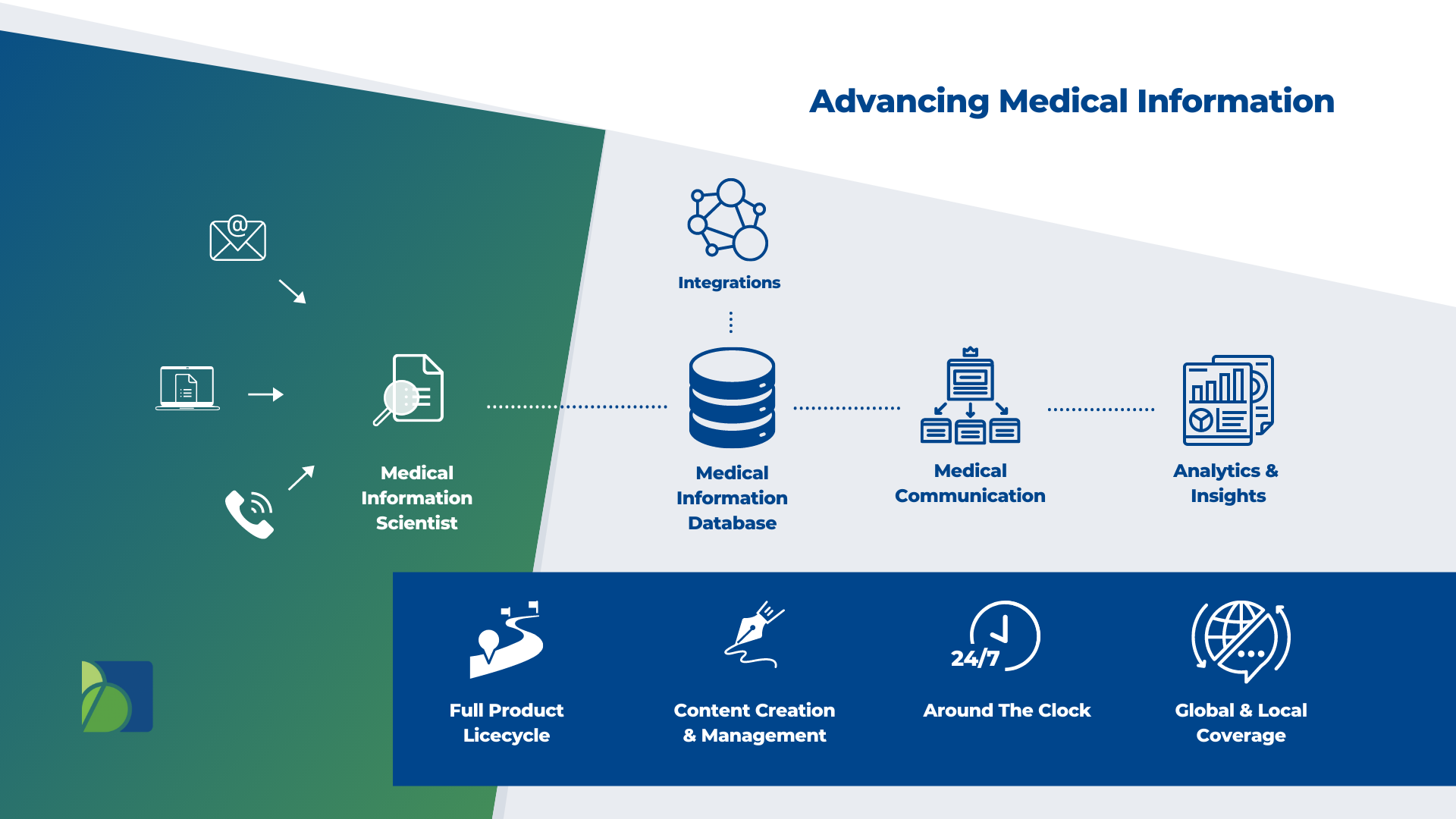
Medical Information Scientist
A 24-hour Medical Information Contact Center is accessible to Biomapas customers seven days a week, 365 days per year. Additionally, our workforce comprises seasoned healthcare professionals and life science graduates who act as an extension of your team. In addition, our experts may assist you with case follow-ups and case completion on your behalf. We go above and above to help your customers and patients in every way that we possibly can.
Medical Information Database
Biomapas Medical Information specialists collecting, manage, and provide real-time oversight of so you can make informed decisions and improved compliance.We streamline our efforts with our adverse event management team and apply with role-based assignment and routing of safety cases for follow-up and medical review.
Medical Communication
Pharmaceutical firms must continuously communicate and educate healthcare professionals to enhance patient care. The traditional definition of Medical Information refers to collecting data, generating the content, responding, and evaluating findings. At Biomapas, Medical Communication represents strategic strategies for presenting and disseminating information to your intended audience.
Analytics & Insights
What value can Medical Information activities provide a business to turn a legal obligation into a competitive advantage? Biomapas' Medical Information services provide enormous insights and results with significant strategic potential for pharmaceutical companies and should be a key partner in designing patient experiences. These insights help recognize emerging themes, indicate early problems, and identify high-impact areas for future medical education.
Integrations
We combine our Medical Information expertise with RA & PV in multi-disciplinary teams. As a result, we can offer our clients a combination of in-house expertise and the tools they need to guarantee patient safety.
Full Product Lifecycle
Since the advent of ATMPs and personalized medications, it has become more challenging to provide Medical Information analytics. Due to a combination of high demand, limited resources and growing difficulties in getting new products on the shelves, operations may be severely impacted by this. We offer efficient solutions to ensure contact centre traffic, triage, and response documents are adequately managed consistently and globally.
Content Creation & Management
Your library of critical documentation can be created entirely or seamlessly added to by Biomapas' medical writing experts, who have extensive expertise in various therapeutic fields and academia.
- Standard Document Creation
- Custom Response and Content Management
- Response Document Maintenance
- Translation and Localization Services
Around The Clock
Our Medical Information staff represents highly-trained experts, which includes multilingual native speakers. In addition, of course, our Contact Center Support is available 24/7/365. Above all, Biomapas aims to put technology first to offer a modern solution that is both high-quality and efficient.
Global & Local Coverage
Because we have local employees throughout all of the European, CIS, EAEU and MENA regions, we have a high level of regional, cultural, and legal knowledge under the Biomapas umbrella. In addition, as a result of our extensive experience in Clinical Research, Regulatory Affairs, Pharmacovigilance, and Medical Information, we can provide comprehensive solutions.
Medical Inquiry Intake & Management
|
Biomapas’ proven global network with profoundly experienced professionals to accurately fulfill inquiries across Europe, CIS/EAEU, and the US are at your disposal.
|
Multilingual Call Handling Service Medical Inquiry Management System Respond to inquiries through both verbal and written communication channels Adverse event, Product Quality Complaint Intake and Handling |
Medical Writing
|
Our Medical Writers can rapidly review or create new content and translate Medical Information documents, including Standard Responses’ Documents update or creation and Custom Response Creation.
|
Quality Management
| Our team exercises their knowledge and experience to ensure high-quality results, create suitable SOPs, set up KPIs and metrics, and always stay focused on compliance. |
Medical Inquiry Intake & Management
Biomapas’ proven global network with profoundly experienced professionals to accurately fulfill inquiries across Europe, CIS/EAEU, and the US are at your disposal.
Multilingual Call Handling Service
Medical Inquiry Management System
Respond to inquiries through both verbal and written communication channels
Adverse event, Product Quality Complaint Intake and Handling
Medical Writing
Our Medical Writers can rapidly review or create new content and translate Medical Information documents, including Standard Responses’ Documents update or creation and Custom Response Creation.
Quality Management
Our team exercises their knowledge and experience to ensure high-quality results, create suitable SOPs, set up KPIs and metrics, and always stay focused on compliance.
Flexibility
Flexible scope of services from End-to-End MI function or partial MI activities
Acting Globally
Our integrated contact centres guarantee a response to medical information inquiries 24/7.

CIS Region
The partner of choice to extend your commercialization activities in CIS countries.
A partner that is dedicated to you and understands the challenges you face.






