Regulatory Affairs
Timely execution of projects isn’t luck. It takes passion and experience.
Local regulatory experts for Pharma, Biotech and Medical Device companies across Europe, CIS/EAEU, and MENA regions from dossier development to full life-cycle management. Our full range of regulatory affairs solutions and services are dedicated to delivering the highest quality support, assistance and regulatory approvals. We follow the needs of our customer and their portfolios, as we work with a wide range of product categories: medicinal products, medical devices, cosmetics, and food supplements. Our Regulatory Affairs team has comprehensive expertise and assures quality and accuracy for each task. All team members have majored in life sciences.
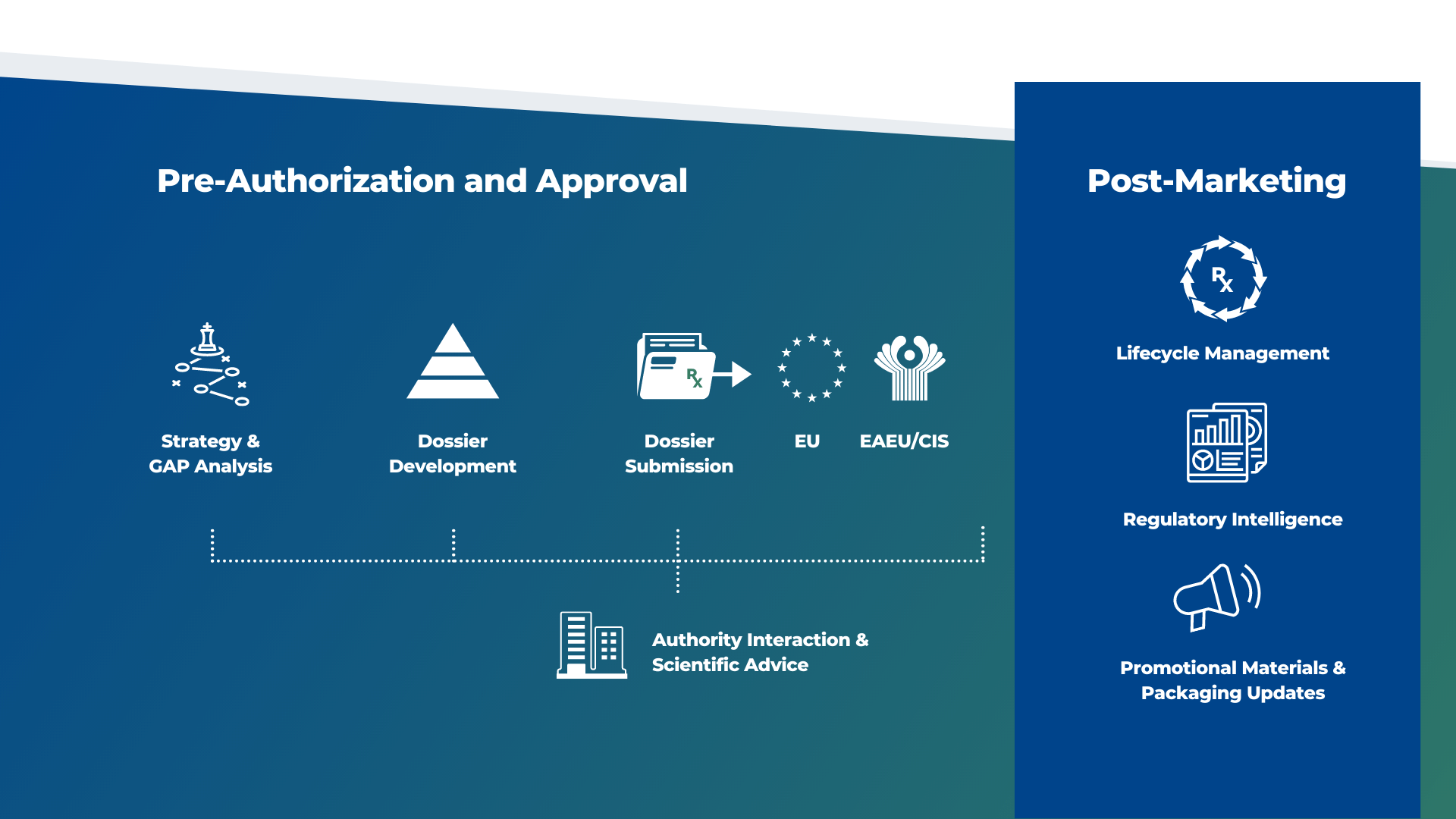
Strategy & Gap Analysis
Having a solid regulatory strategy is essential to ensuring that your product reaches the market in the fastest possible time. With no plan in place, you'll have no way of anticipating potential problems or deadlines. Decades of combined knowledge and local expertise in regulatory consulting means that Biomapas can create a comprehensive strategy. We provide you with a solid strategy, take care of necessary paperwork, and allow you to predict key milestones confidently.
Dossier Development
Scientists with PhDs in biomedical sciences, who have worked in the pharmaceutical business, clinical research organizations, or academic institutions, compose our medical content. In addition, medical Writers from Biomapas may assist you with creating documentation that is needed in clinical research, regulatory affairs, or for scientific reasons, among other things.
Dossier Submission
Throughout Europe, Biomapas offers local regulatory affairs assistance to customers in pre-approval and post-approval regulatory affairs.
It is the goal of our complete spectrum of Regulatory Affairs solutions to provide our customers with an outstanding level of support, help, and regulatory approvals. We use rapid, efficient, and cost-effective working methods to get the job done quickly and efficiently.
EU Support
We have assisted our clients in obtaining more than 150 Marketing Authorizations through National Procedures. Over the past few years, we have executed more than 1,000 regulatory submissions on our clients' behalf. Additionally, we were/are involved in more than 25 marketing authorization processes for medical products, including centralized, decentralized, and mutual recognition procedures.
EAEU Support
If you are interested in marketing your medical product in the Eurasian Economic Union's (EAEU) rapidly expanding pharmaceutical industry, Biomapas is the ideal partner for you. The EAEU is an exciting market for drug registration. Our specialists are eager to discuss the various paths for drug registration in the Eurasian Economic Union and provide guidance on aligning already registered medicines' dossiers with the new criteria.
Authority Interaction & Scientific Advice
As the regulatory environment becomes more complicated, Biomapas' specialists assist our customers in navigating it. We assist you from the planning and implementation of early-stage research through approval and beyond. To help you get the most from Scientific Advice meetings, our experts can prepare questions that deliver the answers you need, interpret agency responses, and rehearse meeting scenarios.
Lifecycle Management
Experience in renewing and modifying pharmaceutical products and other aspects of medical product lifecycle management is essential to achieving corporate objectives. To help you reach them, we offer different solutions to our clients based on their portfolios ranging from small molecules to ATMP's and Medical Devices.
Regulatory Intelligence
With the help of Biomapas' Regulatory Intelligence services, you can create a viable drug development program, conduct efficient trials, and develop a sound commercialization plan. We combine our knowledge and expertise with publically accessible data and experience-based data sources to create a solid strategy that will save you time and money. Shortly, we facilitate you in making strategic decisions and gaining an edge in a competitive market.
Promotional Materials & Packaging Updates
Pharmaceutical firms are required to comply with health authority regulations on promotional materials and activities that they participate in or support with the intent of influencing the prescription and usage of a medicinal product. Therefore, all your promotional materials should comply with the international and national standards established by the European Union (EU) and local health authorities for marketing medicines.
Pre-Authorization
| Biomapas’ expertise ranges from developing and executing your regulatory strategy to pave the way for Marketing Approval. By combining our in-house regulatory expertise with scientific know-how, we help you to ask the right questions and to keep the end goal in mind during early development.
Our focus is on enabling you to navigate the landscape of complicated regulations and compliance hurdles and provide you with a clear strategy for successful commercialization. In addition to complicated regulations and compliance hurdles, we also deliver specific support concerning local needs as they are fundamental to any strategic regulatory decision. As an extension of your team, we cooperate with you every step of the way towards Marketing Authorization. |
Regulatory Strategy / Gap Analysis
Dossier Development/Medical Writing |
Market Authorization
|
Understanding Agency’s timetables for your product’s assessment and having the right people available helps ensure you meet each requirement and beat timelines. Following local laws for countries within Biomapas’ reach, we can prepare initial submission packages and documentation and prepare all country-specific templates. Our support includes collecting all essential documents from the sites and documents received from the Sponsor you need for submissions and acting as the primary, direct contact point with local HAs. An effective project management team that maximizes pace, efficiency, and accuracy of deliverables for the Health Authorities is crucial. Decisions in this phase can determine your product’s future route and affect your marketing and commercialization approach. Comprehension of obtaining the right and formulating effective responses to Agency questions can significantly influence your chances of success. |
Local Regulatory Affairs Support Marketing Authorization Applications National Marketing Authorization Applications
|
Post-Authorization
| Marketed products are continuously subject to change so, Biomapas’ services continue beyond Marketing Authorization. Partnering with you to ensure you meet Post-Authorization requirements regarding your product’s quality and safety throughout its lifecycle.
Our expertise in optimizing regulatory operations around renewals, variations, and other lifecycle management activities is one of the critical elements to reach your business goals
|
Product Lifecycle Management
Local Regulatory Affairs EAEU/CIS Promotional and Advertising Material Review
|
Pre-Authorization
Biomapas’ expertise ranges from developing and executing your regulatory strategy to pave the way for Marketing Approval. By combining our in-house regulatory expertise with scientific know-how, we help you to ask the right questions and to keep the end goal in mind during early development.
Our focus is on enabling you to navigate the landscape of complicated regulations and compliance hurdles and provide you with a clear strategy for successful commercialization. In addition to complicated regulations and compliance hurdles, we also deliver specific support concerning local needs as they are fundamental to any strategic regulatory decision. As an extension of your team, we cooperate with you every step of the way towards Marketing Authorization.
Regulatory Strategy / Gap Analysis
Dossier Development/Medical Writing
Market Authorization
Understanding an Agency’s timetables for your product’s assessment and having the right people available helps ensure you meet each requirement and beat timelines. Following local laws for countries within Biomapas’ reach, we can prepare initial submission packages and documentation and prepare all country-specific templates. Our support includes collecting all essential documents from the sites and documents received from the Sponsor you need for submissions and acting as the primary, direct contact point with local HAs.
An effective project management team that maximizes pace, efficiency, and accuracy of deliverables for the Health Authorities is crucial. Decisions in this phase can determine your product’s future route and affect your marketing and commercialization approach. Comprehension of obtaining the right and formulating effective responses to Agency questions can significantly influence your chances of success.
Local Regulatory Affairs Support
Marketing Authorization Applications
Post-Authorization
Marketed products are continuously subject to change so, Biomapas’ services continue beyond Marketing Authorization. Partnering with you to ensure you meet Post-Authorization requirements regarding your product’s quality and safety throughout its lifecycle.
Our expertise in optimizing regulatory operations around renewals, variations, and other lifecycle management activities is one of the critical elements to reach your business goals
RA Professionals
Countries Covered
%
Of Staff Are Pharmacists
Extension of your team
We aspire to work with you and not for you, complementing capabilities and internal organization.
Inhouse Workforce
We are not another middleman, working through our own workforce across Europe, CIS & MENA

Emerging Markets
The partner of choice to extend your commercialization activities in CIS/EAEU & MENA countries.
Drug Registration in the Eurasian Economic Union (EAEU)
Interested in the EAEU’s fast-growing pharmaceutical market? Learn about the drug registration pathways and aligning previously registered dossiers with the new requirements.
A partner that is dedicated to you and understands the challenges you face.






